Oral BPC-157 vs Injectable: Which Delivery Method Is More Effective?
BPC-157 is one of the few peptides stable enough to survive stomach acid, making oral delivery a real option. Injectable BPC-157 offers higher bioavailability and faster localized effects for injuries like tendon or ligament damage. Oral BPC-157 shines for gut-related research and convenience. Many researchers combine both routes. Your best choice depends entirely on what you are targeting.
Most peptides break down the moment they hit your stomach. That simple fact is why nearly every peptide on the market ships as a lyophilized powder meant for reconstitution and injection. BPC-157 breaks the mold. This pentadecapeptide, derived from a protective protein sequence found in human gastric juice, demonstrates remarkable stability in acidic environments. That stability opens up a question few other peptides face: should you take it orally or inject it?
The answer is not as straightforward as picking one over the other. Each delivery method has distinct advantages backed by different bodies of research. This guide breaks down the evidence, the tradeoffs, and the practical considerations so you can make an informed decision. If you are new to this compound, start with our complete BPC-157 guide for foundational context.
Why BPC-157 Can Survive Your Stomach
Peptides are chains of amino acids, and most of them are fragile. Stomach acid, with a pH hovering between 1.5 and 3.5, rapidly denatures typical peptide bonds. Digestive enzymes like pepsin finish the job. This is precisely why insulin must be injected rather than swallowed.
BPC-157 is different. The acronym stands for Body Protection Compound, and it was originally derived from a protective protein found in human gastric juice (Sikiric et al.). Its 15-amino-acid sequence has evolved to function in exactly the environment that destroys other peptides. Research published in the Journal of Pharmacological Sciences has demonstrated that BPC-157 remains stable in gastric juice for extended periods without significant degradation.
Unlike virtually every other research peptide, BPC-157 resists degradation by stomach acid and digestive enzymes. This inherent stability is not a product of special formulation or enteric coating. It is a fundamental property of the peptide itself, rooted in its origin as a fragment of a gastric protection protein. This is the single factor that makes the oral vs. injectable debate relevant at all.
Oral vs Injectable: Head-to-Head Comparison
| Factor | Oral BPC-157 | Injectable BPC-157 |
|---|---|---|
| Bioavailability | Lower systemic absorption (no published PK data for exact percentage) | Higher systemic absorption typical of subcutaneous peptide delivery |
| Primary Target Tissue | GI tract, gut lining, digestive system | Localized tissue near injection site (tendons, ligaments, muscles) |
| Onset of Action | Gradual, systemic distribution slower | Faster localized effects |
| Convenience | Very high – swallow a capsule or liquid | Low – requires reconstitution, syringes, sterile technique |
| Research Depth | Substantial animal studies, especially GI models | Extensive animal studies across multiple tissue types |
| Cost Per Month | $40-$70/month (capsules) | $50-$90/month (vials + supplies) |
| Skill Required | None | Moderate – proper reconstitution and injection technique |
| Storage | Room temperature (capsules), refrigerate after opening (liquid) | Refrigeration required after reconstitution (shelf life details) |
Research Evidence for Oral BPC-157
The bulk of oral BPC-157 research focuses on gastrointestinal protection and healing. Several key animal studies stand out.
A widely cited study by Sikiric et al. (1999) demonstrated that orally administered BPC-157 significantly accelerated healing of gastric ulcers in rats. The peptide reduced lesion size and promoted angiogenesis in damaged stomach tissue at doses as low as 10 mcg/kg given in drinking water.
Subsequent research explored oral BPC-157 in models of inflammatory bowel disease. In a 2003 study, rats with induced colitis showed marked improvement in tissue damage scores after oral BPC-157 administration over a 14-day period. The researchers observed reduced inflammatory markers and accelerated mucosal repair compared to control groups.
Additional studies have examined oral BPC-157 in models of NSAID-induced gut damage, liver damage from alcohol exposure, and esophageal lesions. Across these models, the consistent finding is that oral BPC-157 exerts strong protective and healing effects on GI tract tissues.
One important note: these studies are preclinical, conducted in rodent models. No large-scale human clinical trials have been published for oral BPC-157. The research is promising but still experimental.
Research Evidence for Injectable BPC-157
Injectable BPC-157 research spans a broader range of tissue types, largely because injection allows higher concentrations to reach non-GI tissues.
A landmark study by Staresinic et al. (2003) demonstrated that intraperitoneally administered BPC-157 accelerated healing of transected Achilles tendons in rats. The treated group showed increased collagen organization and superior biomechanical strength compared to controls.
Research by Cerovecki et al. (2010) extended these findings to ligament injuries. Rats with severed medial collateral ligaments treated with locally injected BPC-157 showed faster functional recovery and improved tissue organization at both macro and microscopic levels.
Other injectable BPC-157 studies have documented effects on muscle healing after crush injuries, bone fracture repair, and even nerve regeneration following transection. A 2010 study showed that BPC-157 injected near damaged peripheral nerves promoted Schwann cell proliferation and accelerated functional nerve recovery.
The injectable route also features research on systemic effects, including studies on blood pressure regulation, nitric oxide pathway modulation, and protection against organ damage. For practical guidance on administration, see our guides on how to inject peptides and subcutaneous vs intramuscular injection.
Bioavailability Breakdown
Bioavailability refers to the percentage of a compound that reaches systemic circulation in active form. This is where the two routes diverge most sharply.
Oral BPC-157: While the peptide survives gastric acid, it still faces absorption barriers in the intestinal wall. No published pharmacokinetic study has quantified exact oral bioavailability in percentage terms. What the research does show is that oral BPC-157 produces measurable systemic effects in animal models, meaning some portion reaches circulation. For GI-targeted applications, systemic bioavailability is less relevant. The peptide acts directly on gut tissue as it passes through, meaning local bioavailability in the GI tract is effectively 100%.
Injectable BPC-157: Subcutaneous injection bypasses the entire digestive system. Bioavailability via subcutaneous injection is generally high for peptides, consistent with established pharmacokinetic principles. The peptide enters the bloodstream quickly and distributes systemically, with higher concentrations near the injection site due to local diffusion.
This difference matters less than you might think if your research focus is gut health. But for anything beyond the GI tract, injectable delivery provides a significantly higher effective dose to target tissues.
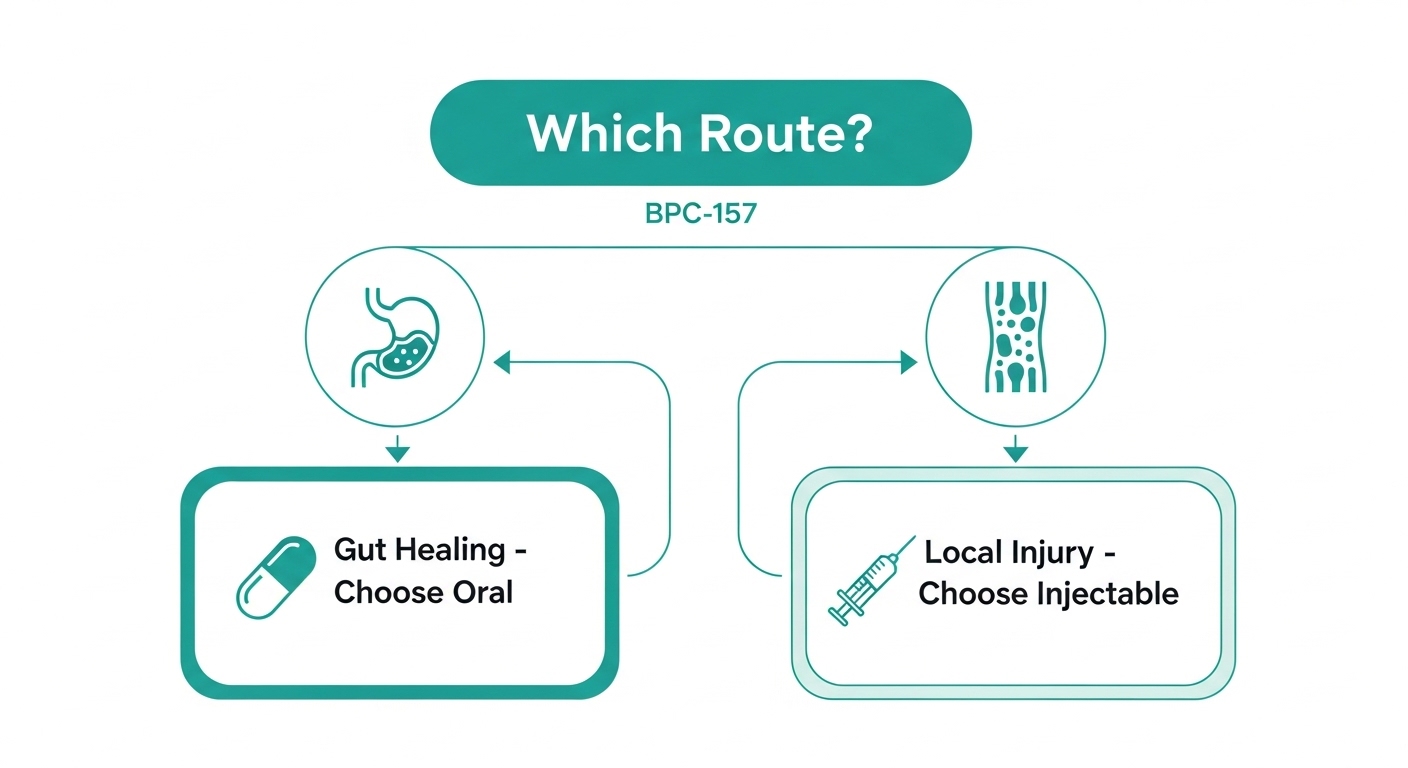
When Oral BPC-157 Makes More Sense
Oral administration has clear advantages in several scenarios.
Gut healing research. If the target is stomach lining, intestinal mucosa, or general GI protection, oral delivery puts the compound exactly where it needs to be. The research supporting oral BPC-157 for ulcers, colitis, and NSAID-induced gut damage is the strongest body of evidence for this route.
Convenience and compliance. Swallowing a capsule requires zero technical skill. There is no need to learn reconstitution, maintain sterile technique, or deal with needles. For anyone uncomfortable with injections, oral BPC-157 removes a major barrier.
Travel and portability. Capsules are shelf-stable, require no refrigeration, and pass through airport security without raising questions. Try traveling with vials, bacteriostatic water, and syringes for comparison.
General maintenance protocols. Some researchers use low-dose oral BPC-157 as a general gut health support alongside other compounds. The convenience makes consistent daily use more practical over longer periods.
When Injectable BPC-157 Makes More Sense
Injection is the stronger choice when you need higher systemic bioavailability or localized tissue effects.
Tendon and ligament injuries. The most compelling injectable BPC-157 research involves musculoskeletal tissue repair. Subcutaneous injection near an injured tendon or ligament delivers a concentrated dose directly to the target area. Oral administration cannot replicate this localized concentration.
Muscle injuries. Similar logic applies to muscle tears or strains. Injecting near the injury site provides direct access to damaged tissue rather than relying on systemic distribution from oral absorption.
Faster onset. Subcutaneous injection delivers the peptide to the bloodstream within minutes. Oral absorption is slower and less predictable. When time matters, injection has the edge.
Dose precision. With injectable BPC-157, you control exactly how many micrograms enter the body. Oral dosing involves more variables, including stomach contents, individual absorption rates, and gut transit time. Researchers who need tight dose control typically prefer injection.
Can You Combine Both Routes?
Some research protocols use both oral and injectable BPC-157 simultaneously. The rationale is straightforward: oral delivery covers GI protection while injectable delivery targets a specific injury site.
A common approach in anecdotal reports involves taking oral BPC-157 daily for gut support while injecting near an injury site for localized repair. There is no published research specifically testing this dual-route protocol, but the mechanism makes theoretical sense. The two routes target different tissues with minimal overlap.
If you consider a combined approach, total daily dosage across both routes should be factored together. Most anecdotal protocols reference total daily research doses in the 250-500 mcg range, split between routes. As always, this is based on animal study extrapolations and community reports, not clinical trials.
Product Forms Available
BPC-157 ships in several forms, each suited to different delivery methods.
Capsules
Pre-measured oral capsules are the simplest option. They typically contain 200-500 mcg of BPC-157 per capsule, sometimes with stabilizing agents. Quality varies significantly between vendors, so sourcing matters. Reputable suppliers like Swiss Chems provide third-party testing certificates.
Liquid Oral Solutions
Some vendors offer BPC-157 in liquid form designed for sublingual or oral use. These allow more flexible dosing than capsules. The liquid is held under the tongue for 60-90 seconds before swallowing, which may improve absorption through the sublingual mucosa.
Lyophilized Powder for Injection
The most common form for researchers. Freeze-dried BPC-157 in sealed vials requires reconstitution with bacteriostatic water before use. This form offers the most flexibility in dosing and the longest shelf life when stored properly. Vendors like Core Peptides and Paradigm Peptides are known for reliable lyophilized products.
Cost Comparison
Pricing varies by vendor, but here is a realistic monthly cost breakdown based on a typical research dose of 250-500 mcg per day.
| Delivery Method | Monthly Cost (Est.) | What Is Included |
|---|---|---|
| Oral Capsules | $40-$70 | 30-60 capsules depending on dose per cap |
| Liquid Oral | $45-$75 | One bottle (typically 30-day supply) |
| Injectable (Lyophilized) | $50-$90 | 1-2 vials (5mg each) + bacteriostatic water + syringes |
Injectable costs include ancillary supplies: bacteriostatic water ($8-$12), insulin syringes ($10-$15 for a box of 100), and alcohol swabs. These add roughly $15-$25 to the total but last multiple months. The peptide itself is often priced similarly across forms on a per-milligram basis. Check our best peptide companies page for current vendor pricing and discount codes.
Related Articles
- The Complete BPC-157 Guide: Benefits, Dosage, and Research
- How to Inject Peptides: A Step-by-Step Guide
- Subcutaneous vs Intramuscular Injection: Which Is Better for Peptides?
- How to Reconstitute Peptides: Complete Instructions
- Do Peptides Expire? Storage and Shelf Life Guide
- Best Peptide Companies: Vendor Rankings and Reviews
Ready to Source Quality BPC-157?
We have tested and reviewed the top peptide vendors so you do not have to guess. Find verified suppliers with third-party testing and fast shipping.
Frequently Asked Questions
Is oral BPC-157 as effective as injectable?
It depends on the target. For gut-related research, oral BPC-157 is well-supported by animal studies and delivers the peptide directly to GI tissue. For musculoskeletal injuries, injectable BPC-157 provides higher localized concentrations and has stronger research backing for those specific tissues.
Does oral BPC-157 actually get absorbed?
Yes. BPC-157 is uniquely resistant to gastric degradation, and preclinical studies confirm systemic effects from oral dosing. However, systemic bioavailability is lower compared to injection, though exact percentages have not been established in published studies.
Can I take BPC-157 orally and inject it at the same time?
Some research protocols use both routes simultaneously. Oral for GI support and injectable for localized tissue repair. There is no published research on this specific combination, but the routes target different areas with distinct mechanisms.
What is the typical oral BPC-157 dosage in research?
Animal studies commonly use doses in the range of 10-50 mcg/kg administered in drinking water. Extrapolated anecdotal human-equivalent protocols typically reference 250-500 mcg per day orally. These are not clinically validated human doses.
Do BPC-157 capsules need to be taken on an empty stomach?
Most anecdotal protocols suggest taking oral BPC-157 on an empty stomach to maximize absorption and reduce interference from food. However, the peptide’s gastric stability means it is not destroyed by stomach acid regardless of food intake.
How long does it take for oral BPC-157 to work?
Animal studies on GI healing typically show measurable effects within 7-14 days of consistent oral dosing. Anecdotal reports from researchers vary widely, with some noting changes within a week and others requiring 3-4 weeks. Individual responses differ significantly.
